NCSS to Participate in the International Forum of Pharmaceutical Inspectorates in Abu Dhabi
23.11.2025
NCSS summarized its participation in the international exhibition Pharmtech & Ingredients 2025
11.12.2025At the Pharmtech & Ingredients Exhibition, the Center Presented a New Strategic Approach to Import Substitution, Combining Development, Technology Transfer, and Regulatory Support.
The National Center of Standard Samples presented new areas of its activities as part of the business program at the Pharmtech & Ingredients 2025 exhibition, including organizing a full cycle for localizing the production of Active Pharmaceutical Ingredients (APIs). This step aims to address one of the industry’s most acute problems – the dependence of Russian drug manufacturers on imported APIs.
During the information session “Quality, Import Substitution, and “Pharma-2030”: New Horizons for NCSS Activities”, the Center’s experts announced their readiness not only to organize the synthesis of key substances but also to ensure a full-scale transfer of technologies to existing production facilities in the Russian Federation. As the speakers emphasized, this process includes a comprehensive analysis of key operational aspects and consultations on regulatory compliance when launching new API production.
This initiative allows manufacturers to reduce risks and accelerate the import substitution process within the state program “Pharma-2030”.
Simultaneously, NCSS announced another significant industry event – the launch of its own production of chromatographic columns. Elena Tereshchuk, Head of Procurement and Sales Department at NCSS, presented test results for the first model, confirming its full compliance with declared characteristics and operational stability on par with leading international analogues. The localization of this key consumable will provide laboratories with affordable prices, prompt deliveries, and guarantee production transparency.
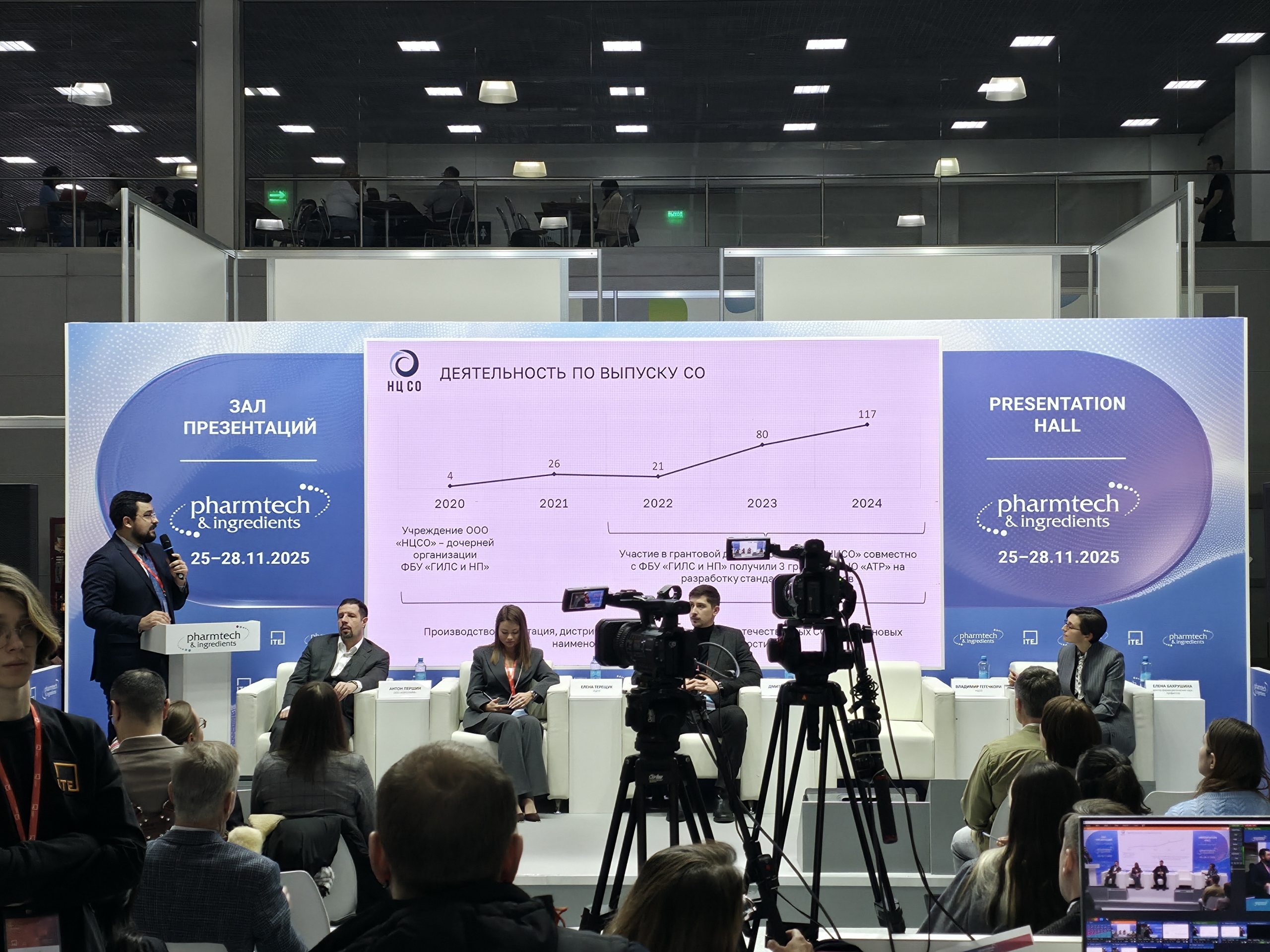
The session also summarized the Center’s five-year work in its primary area – the development and production of certified reference materials (CRMs). Vladimir Gegechkori, Director of Production and Certification, reported that during this time, NCSS has developed 258 certified reference materials, 75 of which have got the status of interstate reference materials. In 2025, he stated, sales of NCSS reference materials showed a twofold increase compared to 2024.
“The use of domestic reference materials reduces economic and logistical risks and directly strengthens the country’s drug security,” he emphasized.
Furthermore, the Center’s team presented comprehensive solution offered by its own certified Testing Laboratory.
The initiatives presented at the exhibition reflect NCSS’ strategic aspiration to expand its activities beyond production of reference materials and become a full-fledged participant in the development of the pharmaceutical industry and the implementation of the “Pharma-2030” state program.